Cement is the single most used material in the world. Despite many centuries of intensive usage and an ever-increasing worldwide demand, many fundamental physio-chemical questions regarding its structure at the nanoscale level remain elusive. During the process of cement setting, different hydrates nucleate and grow, of which calcium silicate hydrate (C-S-H) is the most important binding phase, and is the key compound controlling the final cement properties such as strength and durability.
Although a large deal of research effort has been devoted to the study of C-S-H, its poorly crystalline character has made it inherently difficult to characterise its structural and dynamical properties, and the properties of water adsorbed at its interfaces. Moreover, the presence of aluminate phases both in common Portland cement, and in new low-CO2 emissions cement formulations, results in C-A-S-H, an aluminate-containing C-S-H.
Understanding water organisation in the C-S-H phase is not only important for the setting behaviour, but also because water plays a key role in the processes that are the main causes of cement's strength loss. Moreover, water diffusion in C-S-H nanopores is important to understand when considering other cement applications such as pollutant removal or nuclear waste storage.
In this article, published in Cement and Concrete Research, an international research collaboration studied the dynamics of water in synthetic C-(A)-S-H using inelastic incoherent neutron scattering (IINS) on the Tosca beamline at ISIS and Lagrange at the ILL. They combined these studies with molecular dynamics (MD) simulations and laboratory-based methods such as water sorption isotherms and thermogravimetric analyses. They focused on C-(A)-S-H conditioned at five hydration states (from drier to more hydrated) and with three Ca/Si ratios (0.9, 1, and 1.3).
“IINS is ideal for studying water dynamics because of the large scattering cross section of hydrogen. It enables us to study a wide range of vibrational modes of water in the C-(A)-S-H phase that cannot be measured with other techniques due to selection rules," explains the paper's first author, Zhanar Zhakiyeva from the University of Grenoble Alpes. “This means that using IINS allowed us to probe both the inter- and intra-molecular vibrations,"
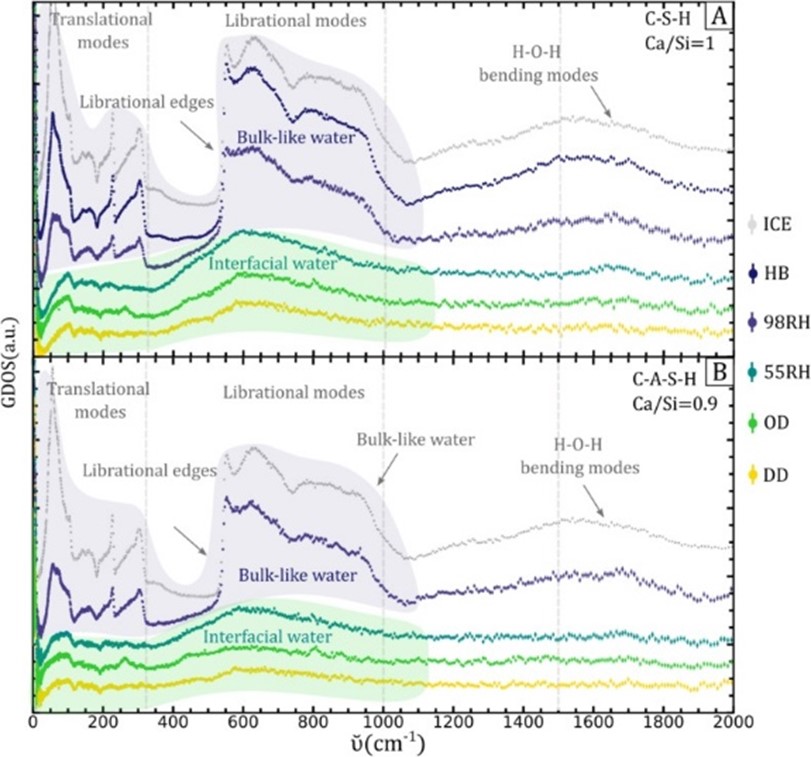 The team interpreted their experimental data with the help of the spectra calculated from their MD models. They observed the characteristic intensity in the 300‑550 cm‑1 region associated with multilayer interfacial water, which turned into ice-like features upon capillary condensation at higher relative humidity. They also saw the so-called “fingerprint" of confined water at lower energies for drier C-(A)-S-H samples. In good accordance with their water sorption measurements in the laboratory, water in C-(A)-S-H samples at higher Ca/Si ratios was found to be more structured and less bulk-water like, due to an increased number of hydrophilic cites created by calcium ions.
The team interpreted their experimental data with the help of the spectra calculated from their MD models. They observed the characteristic intensity in the 300‑550 cm‑1 region associated with multilayer interfacial water, which turned into ice-like features upon capillary condensation at higher relative humidity. They also saw the so-called “fingerprint" of confined water at lower energies for drier C-(A)-S-H samples. In good accordance with their water sorption measurements in the laboratory, water in C-(A)-S-H samples at higher Ca/Si ratios was found to be more structured and less bulk-water like, due to an increased number of hydrophilic cites created by calcium ions.
“Overall, our experimental and simulation results indicate that the evolution from thin layers of interfacial water to bulk-like capillary water is dampened by the structure of C-(A)-S-H," explains Zhanar. “In particular, by the availability of Ca2+ sites that tend to keep the water in the form of structured surface layers. The results from this article complement our previous findings on the structure of water in C-(A)-S-H elucidated by a combination of neutron diffraction, X-ray scattering, and MD simulations."
Determining the range of humidity at which bulk-like water is present in C-(A)-S-H samples is of major importance to understand better some of the mechanisms that take place at, or are controlled by the properties of, C-(A)-S-H – water interfaces. This is the case, for instance, of the carbonation reaction that takes place in the presence of dissolved CO2. It is expected that other processes, such as creep, also can be influenced by the presence of interfacial vs. bulk-like water.
Image caption: The inelastic neutron scattering spectra of C-(A)-S-H (the main binding phase of cement) measured at TOSCA. A) C-S-H and B) C-A-S-H at five hydration states: from containing only interlayer water (yellow) and interfacial water (green), to containing both interfacial and bulk-like capillary water (purple). When dominated by bulk-like water (blue), the spectra are identical to ice Ih (grey).
Related publication: “Water dynamics in calcium silicate hydrates probed by inelastic neutron scattering and molecular dynamics simulations" Cement and Concrete Research, 184, 107616 (2024).
DOI: https://doi.org/10.1016/j.cemconres.2024.107616
Funding: ANR-JCJC 'NUANCE' project (grant ANR-17-CE08-0057). U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Geosciences Program under Award DE-SC0018419. The U.S. Department of Energy, Office of Science, under Award DE-AC02-05CH11231. IDEX mobility scholarship program of the University of Grenoble-Alpes.
Authors: Zhanar Zhakiyeva (Institut Laue-Langevin, France; University of Grenoble Alpes, ISTerre, France; Nazarbayev University, Core Facilities, Kazakhstan), Valérie Magnin, Agnieszka Poulain, Sylvain Campillo, María P. Asta, Rogier Besselink (University of Grenoble Alpes, University of Savoie Mont Blanc, CNRS, IRD, IFSTTAR, ISTerre, France), Stéphane Gaboreau, Francis Claret, Sylvain Grangeon (BRGM, France), Svemir Rudic (ISIS Neutron & Muon Source, STFC-RAL, UK), Stéphane Rols, Mónica Jiménez-Ruiz (Institut Laue-Langevin, France), Ian C. Bourg (CEE department and High Meadows Institute, Princeton University, United States), Alexander E.S. Van Driessche (Department of Mineralogy and Petrology, University of Grenada, Spain), Gabriel J. Cuello (Institut Laue-Langevin, France), Alejandro Fernández-Martínez (University of Grenoble Alpes, University of Savoie Mont Blanc, CNRS, IRD, IFSTTAR, ISTerre, France).
Corresponding Author: Zhanar Zhakiyeva (Institut Laue-Langevin, France; University of Grenoble Alpes, ISTerre, France; Nazarbayev University, Core Facilities, Kazakhstan), Alexander E.S. Van Driessche (Department of Mineralogy and Petrology, University of Grenada, Spain), Alejandro Fernández-Martínez (University of Grenoble Alpes, University of Savoie Mont Blanc, CNRS, IRD, IFSTTAR, ISTerre, France).
