The results, published in Nature, should make it possible to build longer lasting and more cost- and energy-efficient devices such as flow batteries, a promising technology for long-duration grid-scale energy storage.
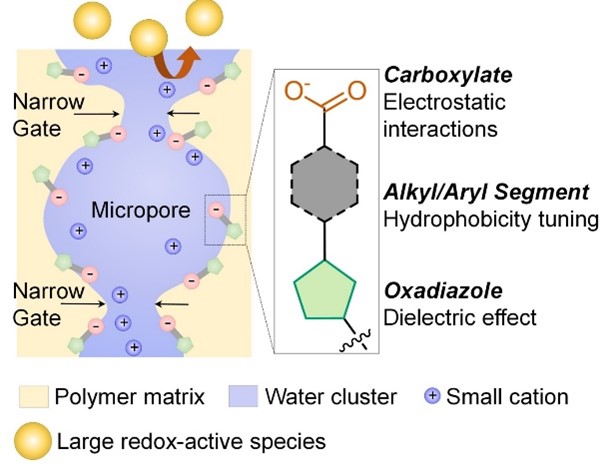
When electric current passes between the electrodes in one of these devices, either going in or going out, it is balanced by an exchange of charged molecules, called ions, within the device. These are generated in liquids, called electrolytes, that surround each electrode. The job of the ion exchange membrane is to separate the two electrodes and their respective electrolytes, while allowing the ions to pass between them.
A good ion exchange membrane will let ions cross rapidly, giving the device greater energy efficiency, while stopping electrolyte molecules in their tracks. Once electrolytes start to leak through, the device gradually loses its effectiveness and its lifespan diminishes. “So the membranes need to be very conductive but also very selective, and this is the key challenge in the field," said Dr Qilei Song from the Department of Chemical Engineering at Imperial College London.
The materials currently used for ion-exchange membranes are far from ideal. The widely used Nafion, for example, is expensive and is manufactured with per- and polyfluoroalkyl substances (PFAS), which are currently being phased out in the USA and Europe because of their toxicity and extreme persistence in the environment. Hence there is a need for alternative sustainable hydrocarbon membranes.
Alternative membranes
Dr Song has been working on a new generation of synthetic polymer membranes for more than a decade. These are based on a class of materials known as polymers of intrinsic microporosity (PIMs), which have rigid and twisted backbones, like fusilli pasta, shot through with tiny pores. These hour-glass shaped micropores provide ordered channels through which small molecules and ions can travel, while the pore gates function like sieves that block large molecules and ions. After Dr Song established his group at Imperial, he revisited the idea of making ion-conductive PIM membranes and, to date, they have developed a series of polymer membranes with a variety of ion-conductive groups, but challenges remain to be solved.
“Studying and controlling membrane swelling has been particularly important, since this can open up the pores and reduce the material's selectivity," said Dr Anqi Wang, the lead author of the latest study, who has been working on this problem through his PhD and postdoc research in Dr Song's group, and who recently became an assistant professor at King Abdullah University of Science and Technology (KAUST) in Saudi Arabia.
“By adding hydrophobic pendant groups to the PIM polymer backbone, such as oxadiazole and benzene rings, we are able to tune the local hydrophobicity and hence tailor the pore size and ion transport when the membrane is in an aqueous solution," he explained.
Understanding water and ion transport
“One scientific question that puzzled us for many years is how the membrane pore structures change at the nano-scale in hydration states, and how ions are transported," said Dr Song, “It was essential to combine molecular modelling and advanced characterisations in order to understand the membrane structures, as well as the water and ion transport dynamics in these membranes."
The team used molecular modelling to study how the membranes swell in water, the effect on pore size, and how ions pass through different locations in the membrane. The modelling was carried out by Charlotte Breakwell, a PhD student in the Department of Chemistry at Imperial, supervised by Professor Kim Jelfs.
“By building the polymer models in hydrated states, we are able to differentiate the tiny change of pore gate sizes upon hydration, which cannot be seen experimentally," said Ms Breakwell. “Furthermore, our quantitative analysis suggested that the ions selectively diffuse through these micropores, and their dynamics are linked with the water diffusion."
“This molecular modelling confirms the effectiveness of tuning the local hydrophobicity around the pores," said Dr Wang. “Now we know our new membrane swells a little, but its pores are still below the one nanometre diameter that we need to maintain selectivity."
Taking a closer look
Based on this result, the team further probed the water and ion dynamics in these sub-nanometre pores. The way that water behaves in the membrane was examined at the nano scale with quasi-elastic neutron scattering (QENS) spectroscopy, carried out by Dr Fabrizia Foglia at University College London using both the IRIS and LET instruments at ISIS, and the BASIS instrument at SNS. Using different instruments enabled them to study multiple samples across a range of timescales.
“With neutron scattering, we are able to differentiate the long-range and local water diffusion within these micropores," said Dr Foglia. “These results suggest a highly interconnected network of water clusters within its large pores, consistent with the molecular dynamics simulation results."
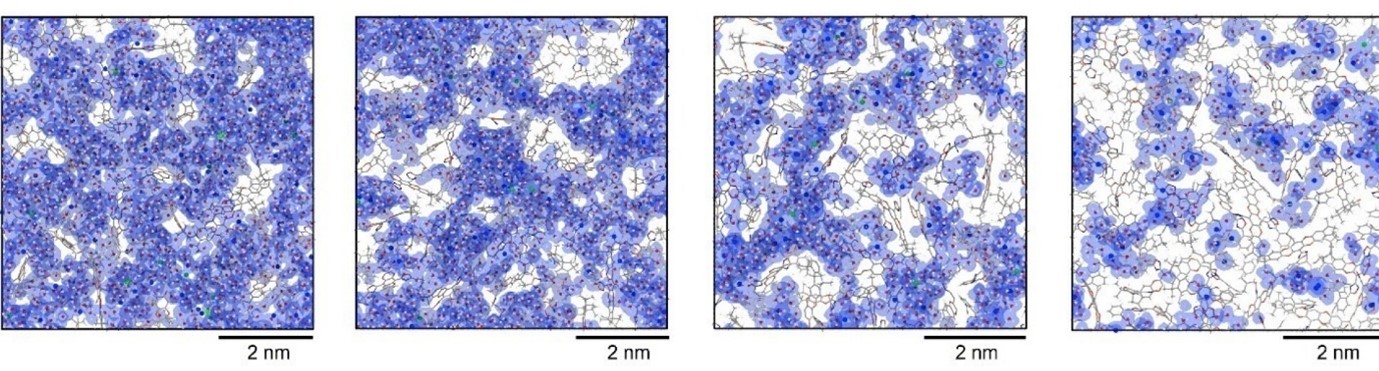
Molecular models of different polymer membranes with micropores less than 2nm
Further information on the water and ion diffusion came from Nuclear Magnetic Resonance studies carried out by Professor Melanie Britton's team at the University of Birmingham. “We are glad to contribute to this work with our expertise in NMR spectroscopy, which effectively probes the dynamics of water and ion transport within these novel membranes," said Professor Britton.
These experimental results were backed up by tests in a redox flow battery, which performed well and proved stable for almost two months. “This is very important, because these batteries are going to be used for large-scale, long-duration green energy storage," Dr Song explained. “You want to operate these batteries for several hours and at the same time have a lifetime of 10 or 20 years, and if the battery decays quickly then it cannot be operated for a long time."
Next steps
Work on the materials is ongoing, with other avenues now being explored for fine-tuning their structure and function. There is also potential to optimise the membrane material for other applications. One line of research focuses on how to further develop these selective membranes for applications such as lithium extraction. This connects with work the group is pursuing on new membrane processes for energy-efficient resource recovery.
“In the future work, we hope to accelerate the discovery of membrane materials by advanced data-driven approaches via machine learning and artificial intelligence," said Professor Jelfs, who is also leading the AI for Chemistry Hub, a joint project between Imperial and the University of Liverpool that is applying machine learning to molecular modelling and the creation of new materials.
The Imperial group has filed a patent on the membrane materials and various aspects of their use. With the support of a UKRI Impact Acceleration grant and a European Research Council Proof-of-Concept grant, the team has also demonstrated the feasibility of using the membranes in a range of devices. “We are currently exploring options for commercialising the technology, and seeking industrial partners to help us develop it further," said Dr Song.
Selective ion transport through hydrated micropores in polymer membranes Anqi Wang, Charlotte Breakwell, Fabrizia Foglia, Rui Tan, Louie Lovell, Xiaochu Wei, Toby Wong, Naiqi Meng, Haodong Li, Andrew Seel, Mona Sarter, Keenan Smith, Alberto Alvarez Fernandez, Mate Furedi, Stefan Guldin, Melanie Britton, Neil McKeown, Kim Jelfs, Qilei Song. Nature, 2024. https://doi.org/10.1038/s41586-024-08140-2.
This article originally appeared on the Imperial College website.
