Gas separations play a crucial role in industrial processes, particularly in capturing impurities, purifying gases and controlling emissions. Though these separations are important, they are often challenging and costly to perform. This is particularly true for separations of gaseous isotopes, such as hydrogen (H2), deuterium (D2), and tritium (T2), which are used in the nuclear industry and are necessary for generation of low-carbon energy from nuclear fusion.
The separation of different hydrogen isotopes is typically complex due to the similarities in their molecular sizes. Cryogenic distillation, which requires huge amounts of energy to cool these gases to extremely low (~20 K) temperatures is the most widely used method of separating these isotopes. Utilising advanced materials for gas separations can result in more sustainable processes, in terms of energy and cost efficiency.
When researchers work with hydrogen isotopes, neutron experiments are invaluable, due to the neutrons' exceptional ability to distinguish between different isotopes.
I am working on hydrogen isotope separation, and neutron techniques are incomparable in detecting hydrogen and its isotopes, as the neutron cross-section of hydrogen is around 40 times2 higher than that of deuterium. Dr Anna Yang, lead author, University of Bristol.
In a recent paper, the group examined the trapdoor behavior of different zeolite frameworks for gas separation applications. The zeolites selected by the Ting group were chosen due to their specialised functional pore windows fitted with trapdoor cations that block the entry of gases into the internal pore cavities and endow them with selective 'trapdoor' gas separation behaviour. The selective adsorption relies on the ability of certain guest molecules to cause a fully reversible deviation of the trapdoor cation from the centre of the pore windows, enabling the selective entry of specific molecules like CO2. This feature offers sophisticated solutions for challenging separations, with trapdoor zeolites serving as promising materials for efficient and specific gas separations, such as the separation of CO2 from N2.
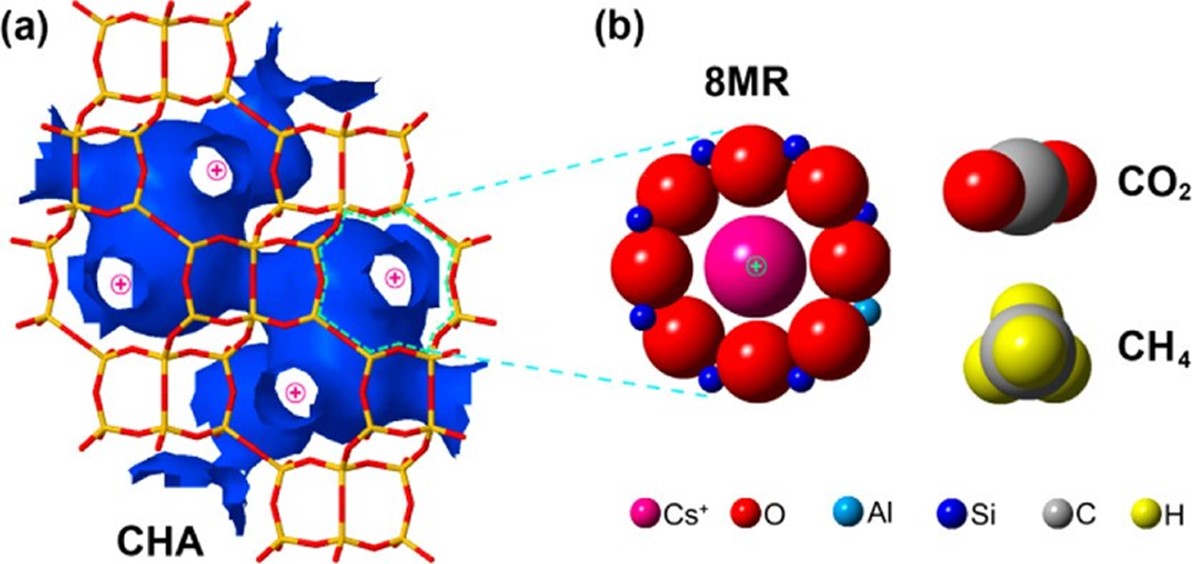
Figure 1: Schematic illustration of the chabazite-gas system.
(a) The framework of the chabazite with its pore windows as the only access to the crystal interior.
(b) Represents one of the pore windows with a ''door-keeping'' cesium cation.
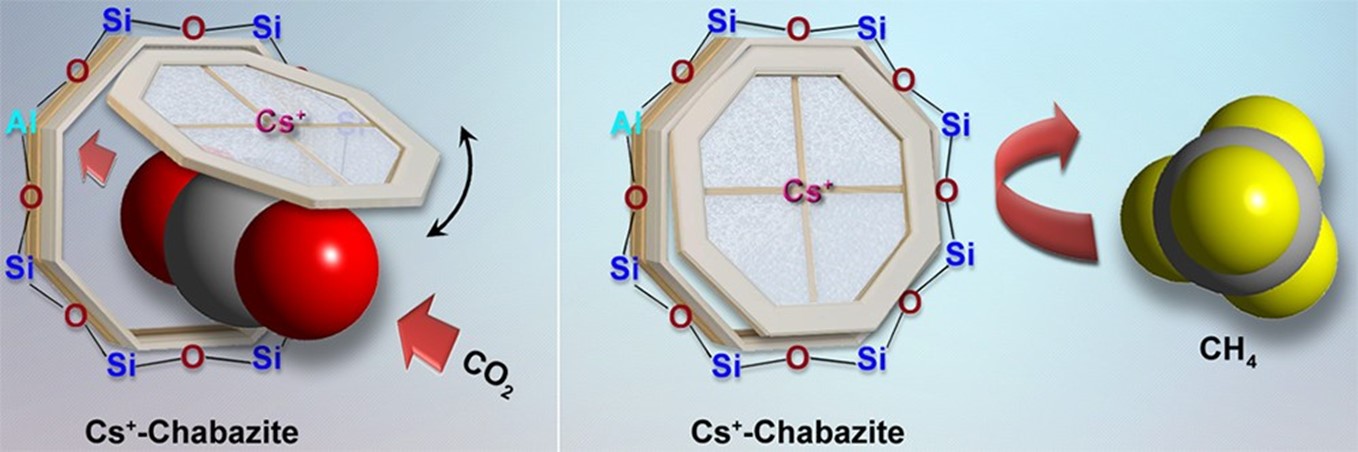
Figure 2: CO2 molecules can open the Caesium trapdoor to enter the chabazite cages whereas CH4 is excluded. After the entry of a CO2 molecule through the pore window, the 'trapdoor' immediately recloses.
The paper examined suitable trapdoor zeolite candidates in different sorption applications by showing the influence of the functional cation and zeolite framework on sample porosities, thermal behaviour, and gas separation ability. The research further extended the understanding of the influence of thermally dependent trapdoor behaviour in gas sorption and proved the interactions between the gases and the trapdoor are stronger than the thermal influence on the structure by in-situ Powder X-ray Diffraction (PXRD). This new understanding can help in the application of such trapdoor zeolites in different gas separations.
To complement the gas separation studies, the ISIS Neutron and Muon Source was utilised for radiation testing, to estimate the stability of the zeolites under extreme conditions for gaseous separation or storage of hydrogen isotopes. In a collaboration with Professor Paul Henry from ISIS, the test conditions were designed to replicate the radiation such materials might experience during application in a nuclear fusion plant, using a sealed, filtered Cs-137 pure gamma-ray source which approximated the type and flux of radiation expected. The results revealed a high tolerance of these materials to radiation, indicating the separation of radioactive tritium may be achieved without damage to the crystalline structure. This could make these materials appropriate for separation of radioactive tritium from helium in nuclear applications.
From these encouraging results, the group plans to further investigate the application of zeolites and other porous materials to challenges in gas separation or storage for nuclear fusion, hydrogen storage and other sustainable technologies.
The full paper can be found at DOI: 10.1021/acs.langmuir.4c00498
