Ever since the creation of insulin in 1922, protein-based drugs have become commonplace in the pharmaceutical industry. However, new more complex protein-based drugs have complications with being stable in the varying environments in the human body and for a long period of time during storage. Sugars could be the solution to this.
Disaccharides, also known as double sugars, are made from two simple sugar molecules and have been found to improve the stabilisation of a variety of proteins. The ability to stabilise across varying pHs and temperatures is due to disaccharides bioprotective properties - the mechanism of which is not known. Disaccharides are currently used in the stabilisation of sperm or hepatocytes during cryopreservation or to prolong the life of an enzyme by reducing the risk of protein denaturation at high temperatures. The most effective disaccharide for stabilisation is trehalose. This is a naturally occurring disaccharide found in a range of organisms from mushrooms to shrimps to bees. In insects, this is a major component of the haemolymph (like our ‘blood’) and acts as store of energy. Protein based drugs need to be kept stable during the storage and transport stage. Greater understanding of the mechanism of disaccharides, in particular trehalose, and their interaction with proteins will help improve protein drug stability.
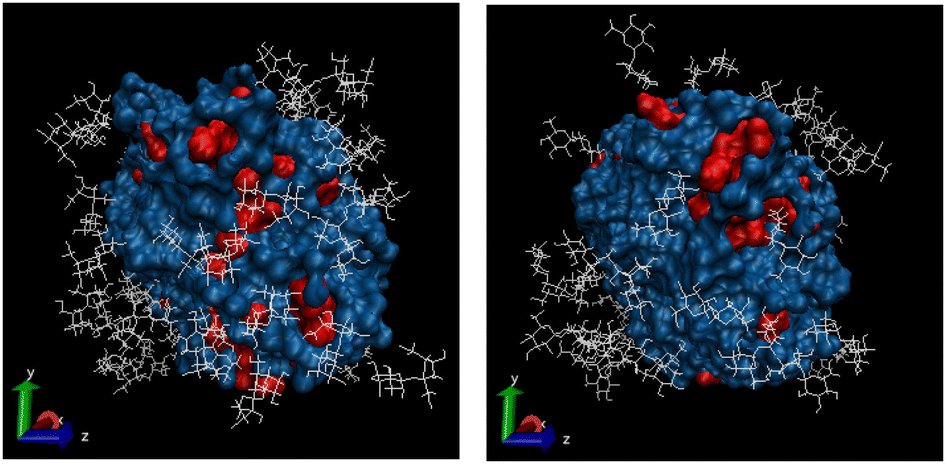
3D image of the ESPR produced structural model of the three-component system with sucrose.
Researchers from the Chalmers University of Technology and KTH Royal Institute of Technology came to ISIS to understand the reasoning behind why trehalose is the best disaccharide to use for stabilising proteins. There have been many previous hypotheses to answer this question, however, these have not been supported by experiments or computational analysis.
Experimental and computational methods were used to observe the dynamics and structural properties of trehalose and sucrose aqueous solution and their effect on myoglobin protein. At ISIS, Neutron Diffraction data was collected using NIMROD and quasielastic neutron scattering (QUENS) was performed using the IRIS spectrometer at 300K.
Small but significant differences between the two disaccharides were discovered. For both sugars, preferential hydration effect is seen. This means the myoglobin prefers to interact with the water (be hydrated) rather than the sugars and the natural hydration shell around the protein is maintained. However, trehalose results in slower protein dynamics than sucrose and has less direct interactions (hydrogen bonds) with the Myoglobin surface. Less interactions suggests that the protein maintains its hydration shell better with trehalose compared to sucrose and hence a greater preferential hydration effect is seen.
This research offers an explanation behind trehalose superiority when it comes to protein stability. It also demonstrates the dynamic interactions between disaccharides and protein and the structures that form as a result. The researchers are currently working on a deeper understanding of the behaviour of disaccharides with other biomolecules for pharmaceutical applications.
The full publication can be found at DOI: 10.1039/D3CP02639F
